🥚 Floating Egg Experiment
Fresh Water vs Salt Water
🧪 What is the experiment?
Put an egg in:
1️⃣ Fresh water
2️⃣ Salt water
See what happens!
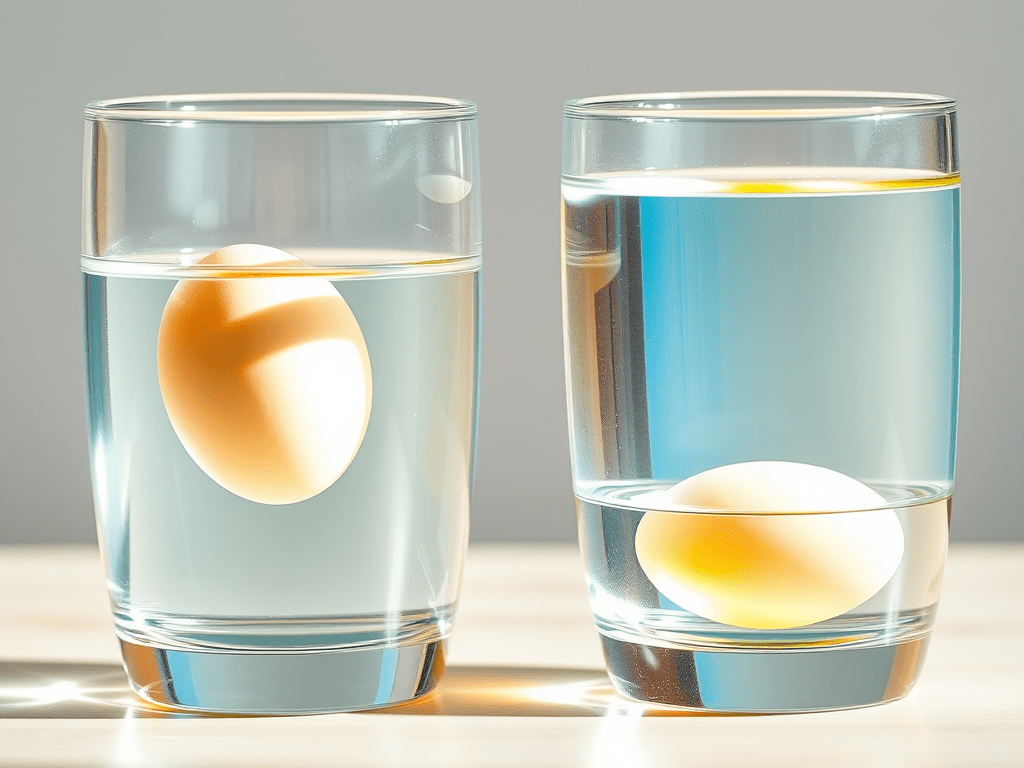
👀 What do you see?
- 🥚 In fresh water → the egg sinks
- 🥚 In salt water → the egg floats
Why does this happen? Let’s find out 👇
🧠 The science behind it (easy way)
🌊 Density (big word, simple meaning)
Density means:
How tightly packed something is
- Heavy and tightly packed → sinks
- Light or spread out → floats
💧 Fresh water
- Fresh water is less dense
- The egg is heavier than fresh water
👉 So the egg sinks
🧂 Salt water
- Adding salt makes water denser
- Salt water becomes heavier than the egg
👉 So the egg floats
⚖️ Think like this:
- Fresh water = thin soup 🍲
- Salt water = thick soup 🍜
Egg floats better in thicker water!
🧪 How to do the experiment at home
- Take 2 glasses of water
- Add salt to one glass and stir well
- Gently drop an egg in each glass
- Observe the difference 👀
🧠 Easy to remember
More salt → more density → more floating

🌊 Real-life connection
- People float more easily in the sea than in a swimming pool
- That’s because sea water has salt!
⭐ Fun facts
- This is why it’s easy to float in the Dead Sea
- Submarines use density to sink and float

Leave a comment